All of Medytox starts from R&D.
Pipeline
R&D is a starting point of drug development, commercialization and a locomotive in realizing continuous growth.
To keep our pride of R&D-based biopharmaceutical company, Medytox invests more than 15% of the total sales in R&D – Especially with Gwanggyo R&D Center, established with an one-stop system to conduct all drug development processes except for clinical trial, we are developing biopharmaceuticals that will pioneer the global market.
To keep our pride of R&D-based biopharmaceutical company, Medytox invests more than 15% of the total sales in R&D – Especially with Gwanggyo R&D Center, established with an one-stop system to conduct all drug development processes except for clinical trial, we are developing biopharmaceuticals that will pioneer the global market.
01
Candidate Selection
Securing
competitive technology /
substances
competitive technology /
substances
02
Nonclinical Study
Cell& animal
based efficacy
and toxicity test
based efficacy
and toxicity test
03
Clinical study
Administration
dose selection &
therapeutic validation
dose selection &
therapeutic validation
04
Approval
Product Approval


Biopharmaceutical
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
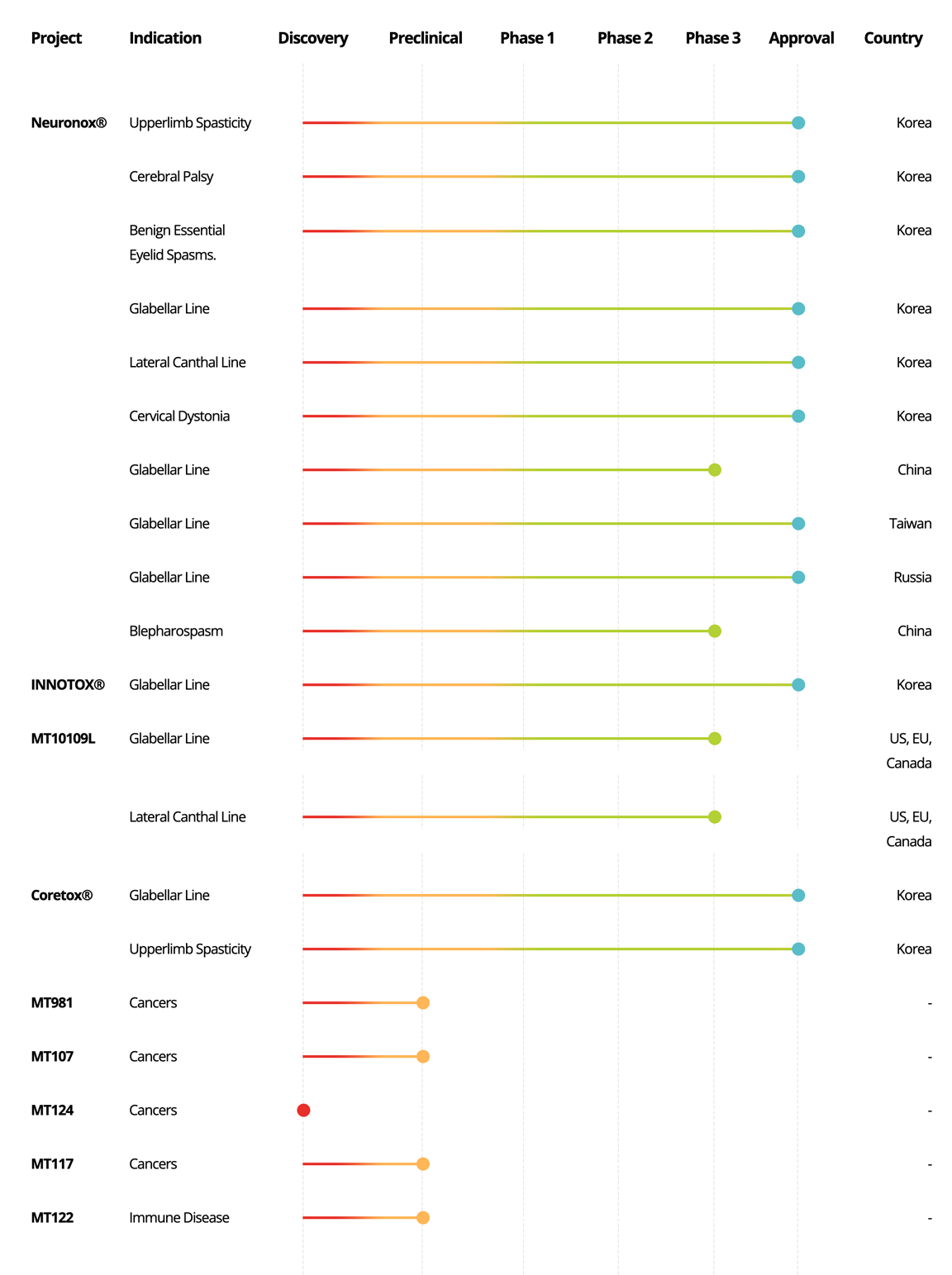
Neuronox®
Upperlimb Spasticity
Korea
Cerebral Palsy
Korea
Benign Essential
Eyelid Spasms.
Eyelid Spasms.
Korea
Glabellar Line
Korea
Lateral Canthal Line
Korea
Cervical Dystonia
Korea
Glabellar Line
China
Glabellar Line
Taiwan
Glabellar Line
Russia
Blepharospasm
China
INNOTOX®
Glabellar Line
Korea
MT10109L
Glabellar Line
US, EU, Canada
Lateral Canthal Line
US, EU, Canada
Coretox®
Glabellar Line
Korea
Upperlimb Spasticity
Korea
MT981
Cancers
-
MT107
Cancers
-
MT124
Cancers
-
MT117
Cancers
-
MT122
Immune Disease
-
Chemical Drug
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
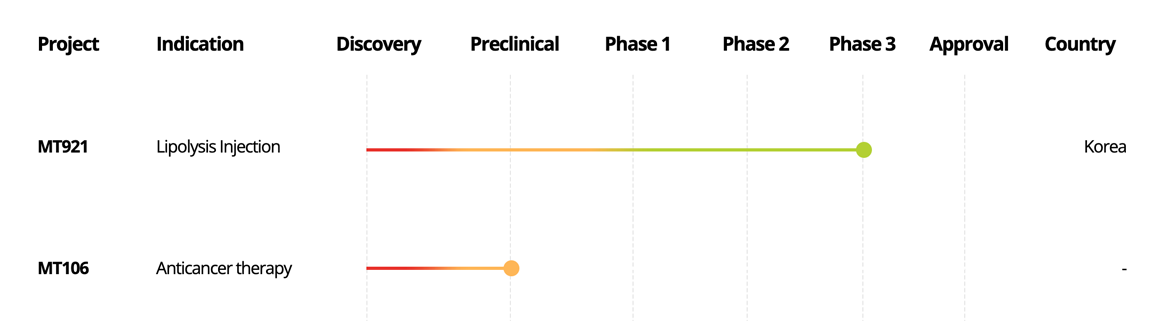
NEWV®
(MT921)
(MT921)
Lipolysis Injection
Korea
MT106
Anticancer therapy
-
Medical Device
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
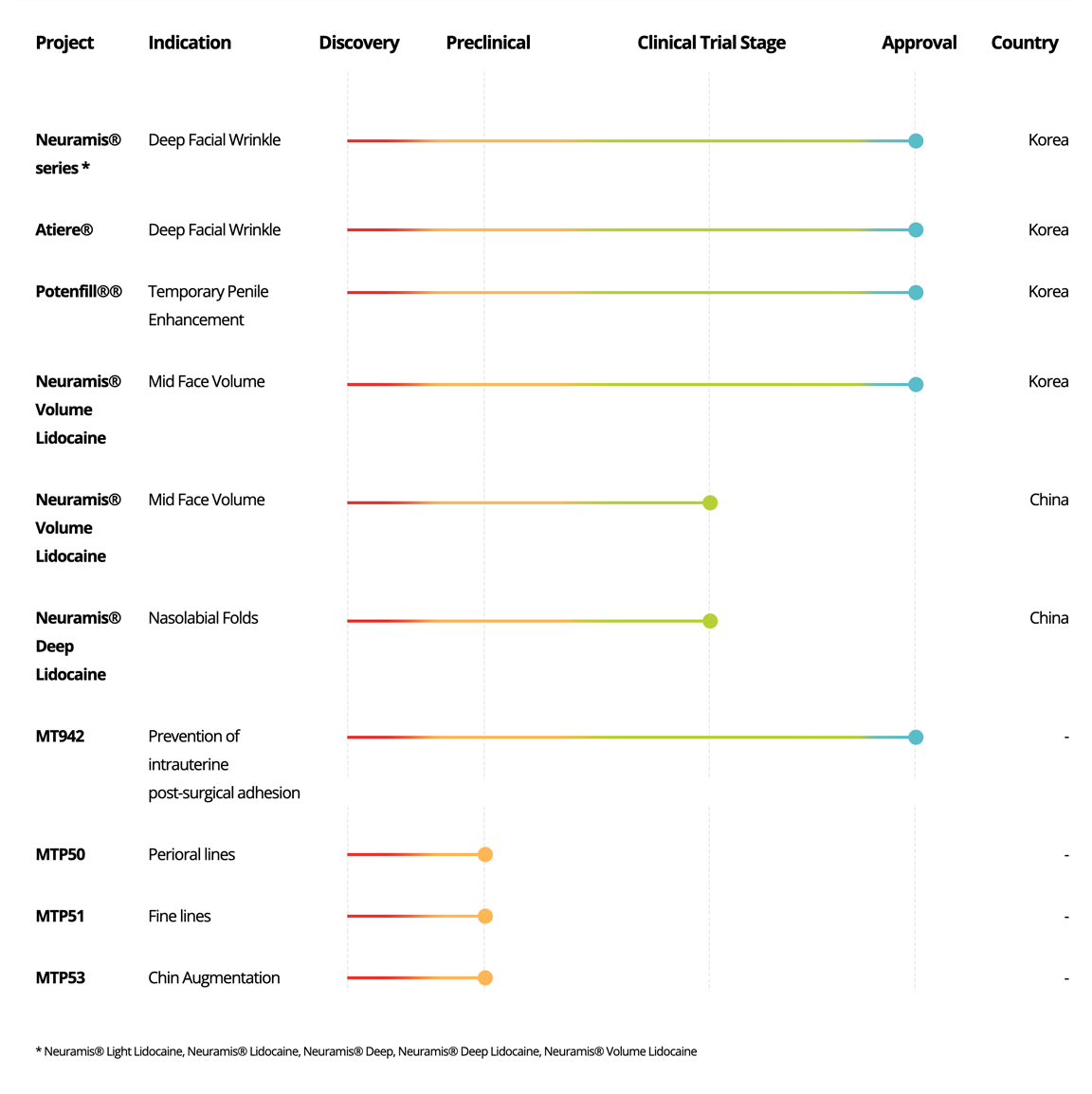
Neuramis®
series *
series *
Deep Facial Wrinkle
Korea
Atiere®
Deep Facial Wrinkle
Korea
Potenfill®®
Temporary Penile
Enhancement
Enhancement
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
China
Neuramis®
Deep
Lidocaine
Deep
Lidocaine
Nasolabial Folds
China
MT942
Prevention of intrauterine
post-surgical adhesion
post-surgical adhesion
-
MTP51
Fine lines
-
MTP53
Chin Augmentation
-
MTP55
Lip Augmentation
-
* Neuramis® Light Lidocaine, Neuramis® Lidocaine, Neuramis® Deep, Neuramis® Deep Lidocaine, Neuramis® Volume Lidocaine
Health Supplements
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
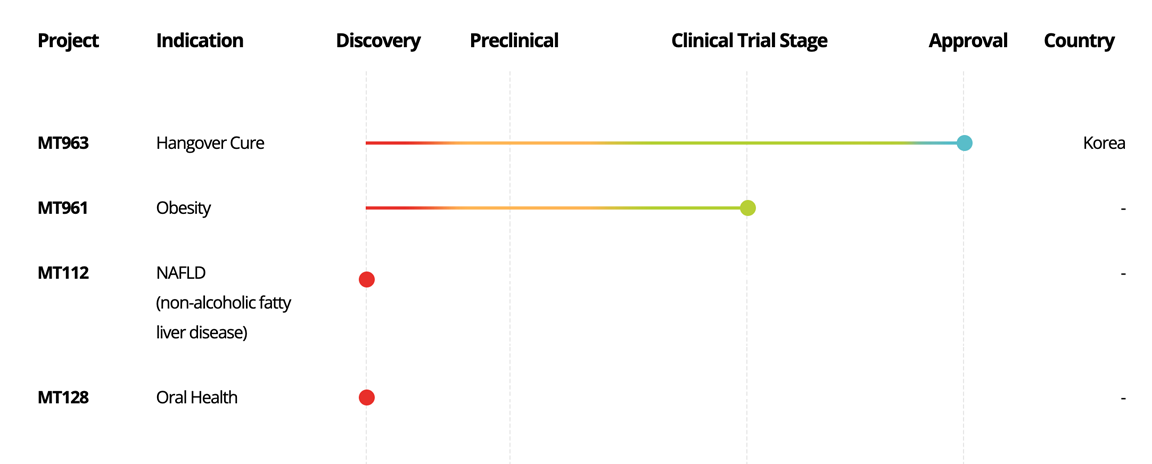
MT963
Hangover Cure
Korea
MT961
Obesity
-
MT112
NAFLD
(non-alcoholic fatty
liver disease)
(non-alcoholic fatty
liver disease)
-
MT128
Oral Health
-