Medytox的所有产品均从研发起步。
研发管道
研发是药物研制和商业化的起点,也是实现持续增长的原动力所在。
作为一家名副其实的科研导向生物制药公司,Medytox每年的研发投资占总销售额比例超过15% – 尤其是在光教研发中心,该中心建有一套一站式体系,用于开展临床以外的全部药物研发流程,目前,我们正在开发足以领先全球市场的生物药剂。
作为一家名副其实的科研导向生物制药公司,Medytox每年的研发投资占总销售额比例超过15% – 尤其是在光教研发中心,该中心建有一套一站式体系,用于开展临床以外的全部药物研发流程,目前,我们正在开发足以领先全球市场的生物药剂。
01
挑选受试者
确保有竞争
力的技术
/ 确保成分
力的技术
/ 确保成分
02
非临床研究
基于细胞
和动物的
药效与毒性测试
和动物的
药效与毒性测试
03
临床研究
用法用量选择
与疗效验证
与疗效验证
04
审批
产品审批


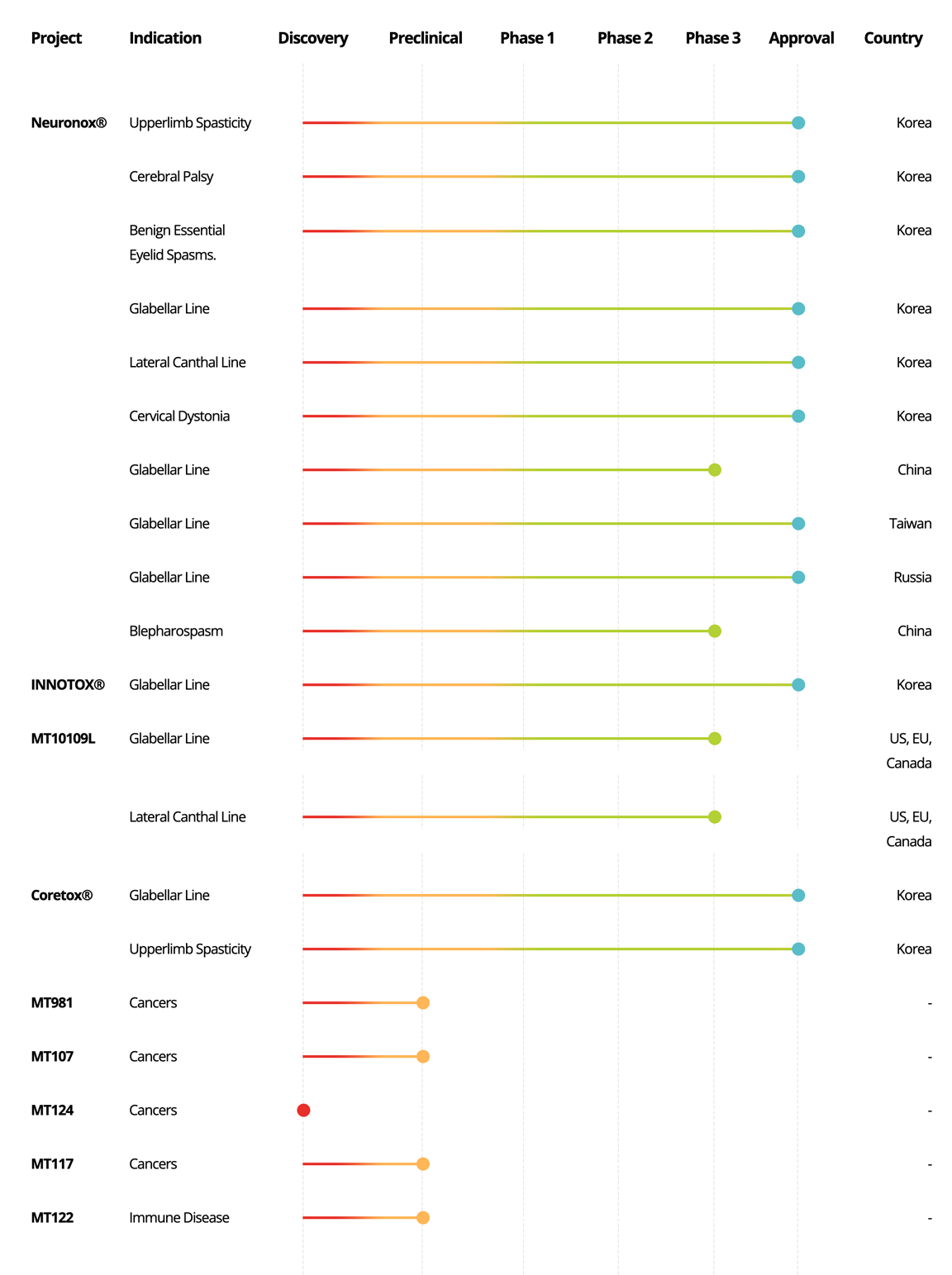
Biopharmaceutical
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
Neuronox®
Upperlimb Spasticity
Korea
Cerebral Palsy
Korea
Benign Essential
Eyelid Spasms.
Eyelid Spasms.
Korea
Glabellar Line
Korea
Lateral Canthal Line
Korea
Cervical Dystonia
Korea
Glabellar Line
Taiwan
Glabellar Line
Russia
INNOTOX®
Glabellar Line
Korea
MT10109L
Glabellar Line
US, EU, Canada
Lateral Canthal Line
US, EU, Canada
Coretox®
Glabellar Line
Korea
Upperlimb Spasticity
Korea
PF30
Glabellar Line
-
MT951
Aesthetic / Treatment
-
MT124
Cancers
-
MT117
Cancers
-
MT122
Immune Disease
-
MT133
Cancers
-
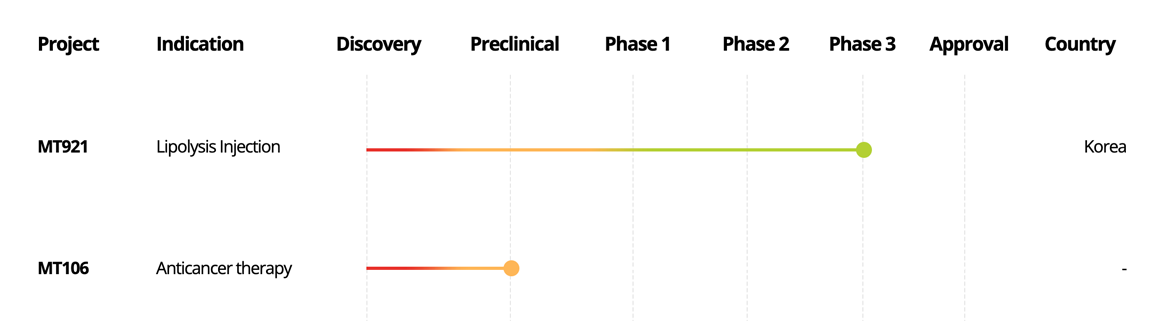
Chemical Drug
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
NUVIJU
(MT921)
(MT921)
Lipolysis Injection
Korea
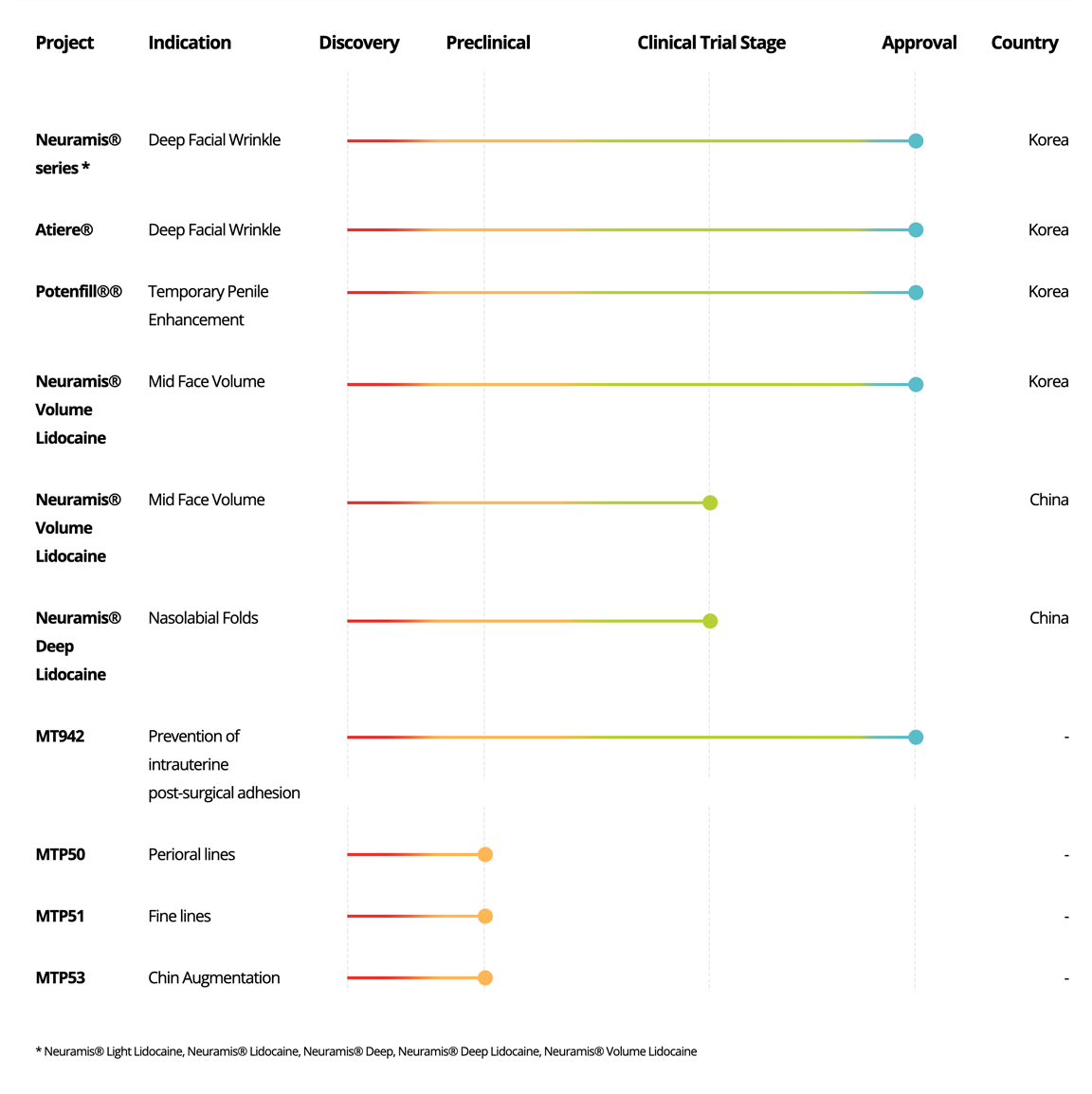
Medical Device
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
Neuramis®
series *
series *
Deep Facial Wrinkle
Korea
Atiere®
Deep Facial Wrinkle
Korea
Potenfill®®
Temporary Penile
Enhancement
Enhancement
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
China
Neuramis®
Deep
Lidocaine
Deep
Lidocaine
Nasolabial Folds
China
MT942
Prevention of intrauterine
post-surgical adhesion
post-surgical adhesion
-
MTP51
Fine lines
-
MTP55
Lip Augmentation
-
MTP57
Infraorbital Hollow
-
MTP58
Fine lines
-
MTP59
Mid Face Volume
-
* Neuramis® Light Lidocaine, Neuramis® Lidocaine, Neuramis® Deep, Neuramis® Deep Lidocaine, Neuramis® Volume Lidocaine
Health Supplements
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
MT963
Hangover Cure
Korea
MT961
Obesity
-








