Medytox的所有产品均从研发起步。
研发管道
研发是药物研制和商业化的起点,也是实现持续增长的原动力所在。
作为一家名副其实的科研导向生物制药公司,Medytox每年的研发投资占总销售额比例超过15% – 尤其是在光教研发中心,该中心建有一套一站式体系,用于开展临床以外的全部药物研发流程,目前,我们正在开发足以领先全球市场的生物药剂。
作为一家名副其实的科研导向生物制药公司,Medytox每年的研发投资占总销售额比例超过15% – 尤其是在光教研发中心,该中心建有一套一站式体系,用于开展临床以外的全部药物研发流程,目前,我们正在开发足以领先全球市场的生物药剂。
01
挑选受试者
确保有竞争
力的技术
/ 确保成分
力的技术
/ 确保成分
02
非临床研究
基于细胞
和动物的
药效与毒性测试
和动物的
药效与毒性测试
03
临床研究
用法用量选择
与疗效验证
与疗效验证
04
审批
产品审批


Biopharmaceutical
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
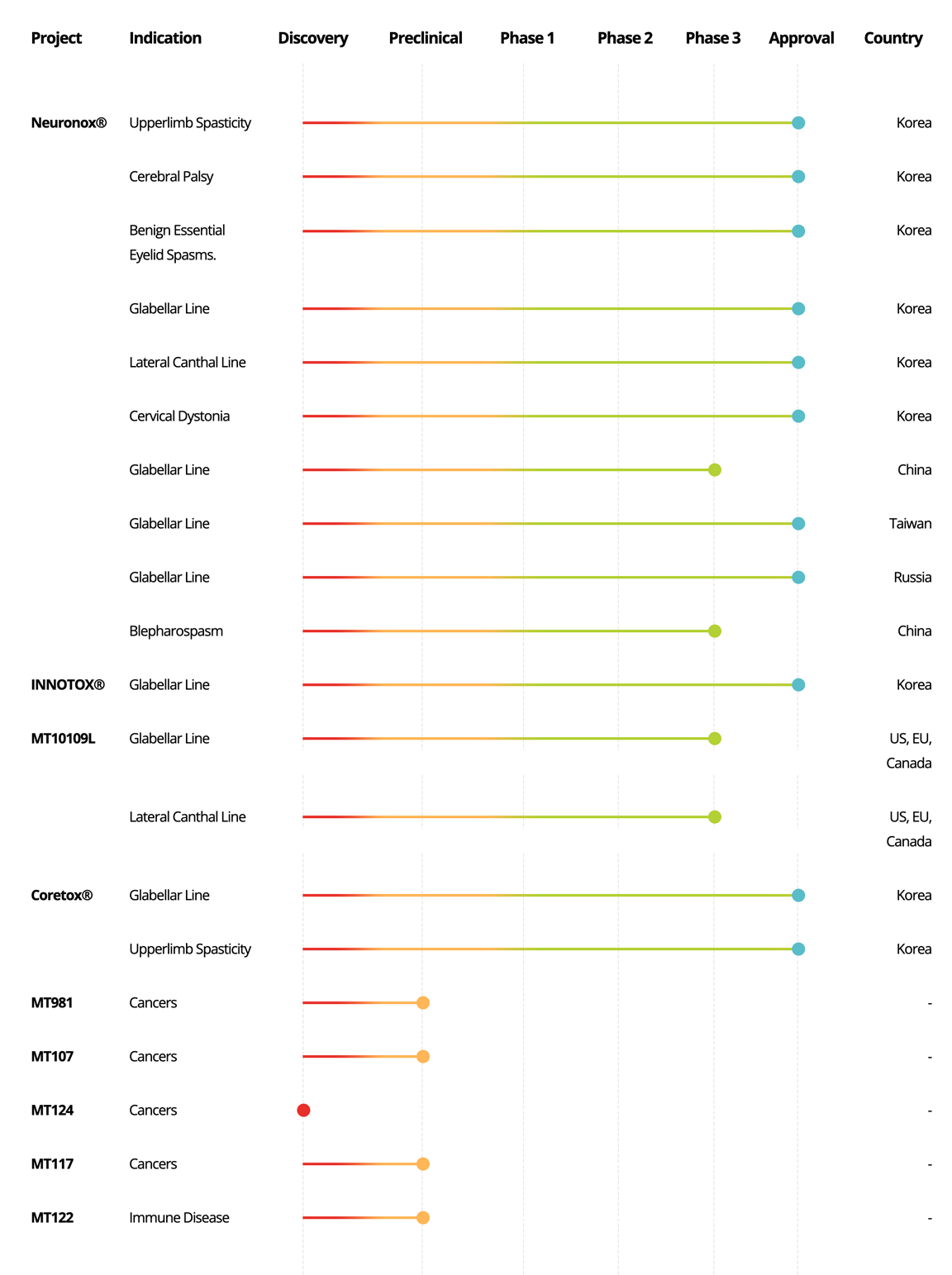
Neuronox®
Upperlimb Spasticity
Korea
Cerebral Palsy
Korea
Benign Essential
Eyelid Spasms.
Eyelid Spasms.
Korea
Glabellar Line
Korea
Lateral Canthal Line
Korea
Cervical Dystonia
Korea
Glabellar Line
China
Glabellar Line
Taiwan
Glabellar Line
Russia
Blepharospasm
China
INNOTOX®
Glabellar Line
Korea
MT10109L
Glabellar Line
US, EU, Canada
Lateral Canthal Line
US, EU, Canada
Coretox®
Glabellar Line
Korea
Upperlimb Spasticity
Korea
MT981
Cancers
-
MT107
Cancers
-
MT124
Cancers
-
MT117
Cancers
-
MT122
Immune Disease
-
Chemical Drug
Project
Indication
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Approval
Country
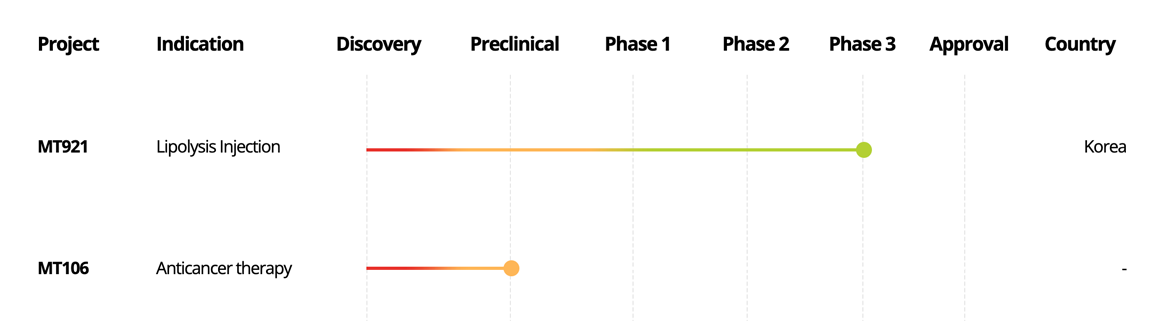
NEWV®
(MT921)
(MT921)
Lipolysis Injection
Korea
MT106
Anticancer therapy
-
Medical Device
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
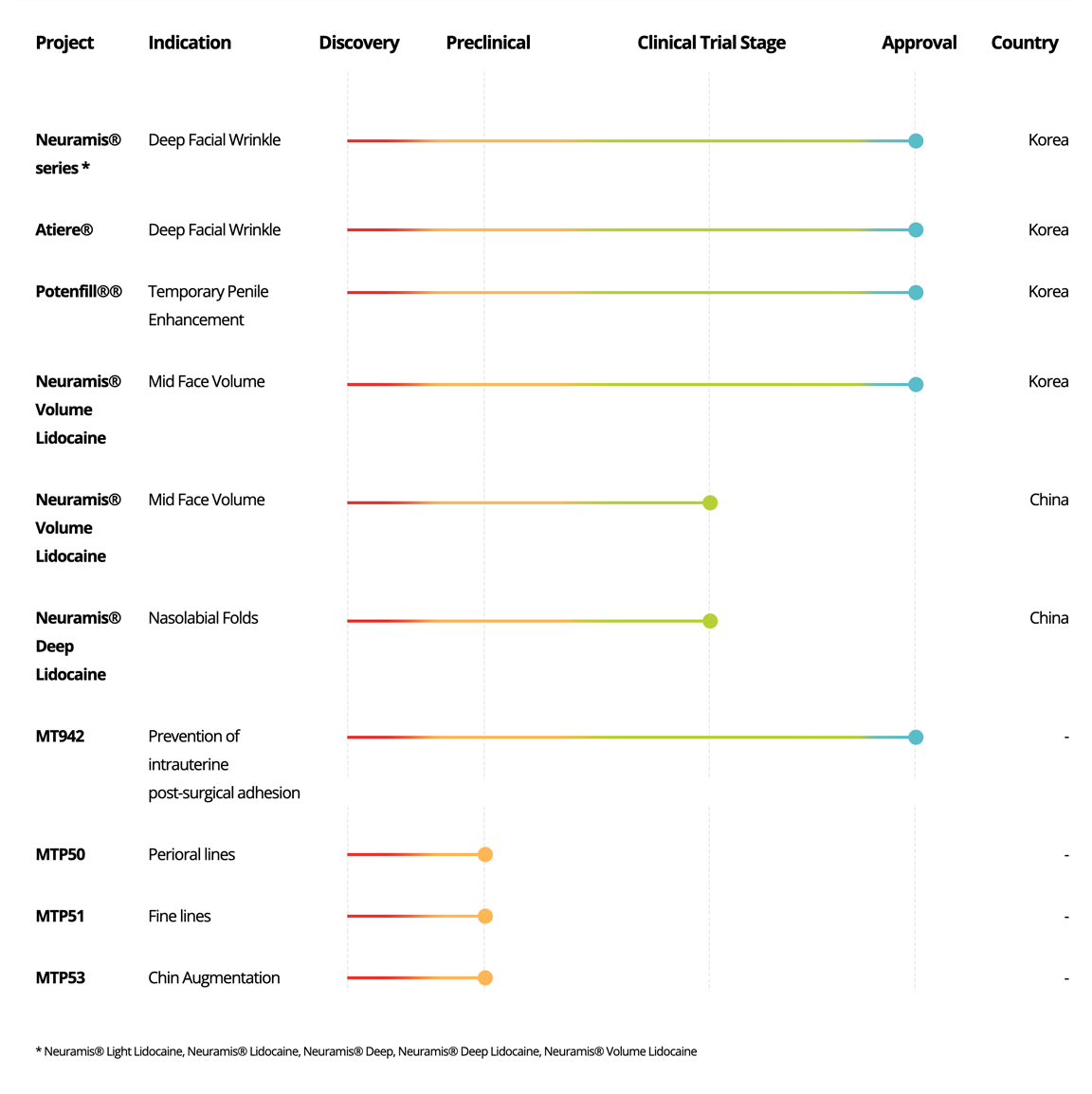
Neuramis®
series *
series *
Deep Facial Wrinkle
Korea
Atiere®
Deep Facial Wrinkle
Korea
Potenfill®®
Temporary Penile
Enhancement
Enhancement
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
Korea
Neuramis®
Volume
Lidocaine
Volume
Lidocaine
Mid Face Volume
China
Neuramis®
Deep
Lidocaine
Deep
Lidocaine
Nasolabial Folds
China
MT942
Prevention of intrauterine
post-surgical adhesion
post-surgical adhesion
-
MTP51
Fine lines
-
MTP53
Chin Augmentation
-
MTP55
Lip Augmentation
-
* Neuramis® Light Lidocaine, Neuramis® Lidocaine, Neuramis® Deep, Neuramis® Deep Lidocaine, Neuramis® Volume Lidocaine
Health Supplements
Project
Indication
Discovery
Preclinical
Clinical Trial Stage
Approval
Country
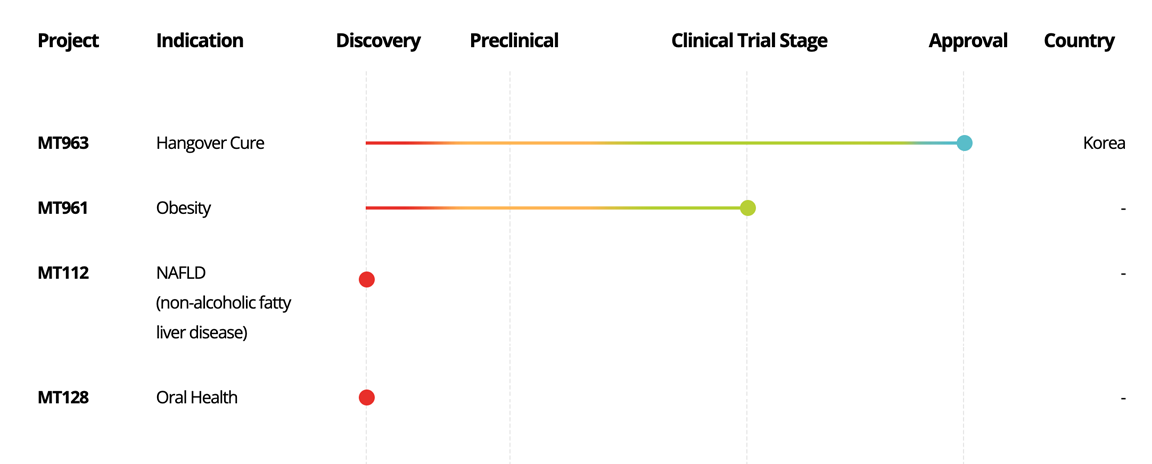
MT963
Hangover Cure
Korea
MT961
Obesity
-
MT112
NAFLD
(non-alcoholic fatty
liver disease)
(non-alcoholic fatty
liver disease)
-
MT128
Oral Health
-